NuVasive (NUVA) Launches Modulus ALIF for Anterior Spine Surgery
NuVasive, Inc. NUVA recently announced the commercial launch of Modulus ALIF, a 3D-printed porous titanium implant for anterior lumbar interbody fusion (ALIF), in targeted global regions. The Modulus ALIF is considered to be one of NuVasive's most successful clinical evaluations to date, with continued surgeon adoption and overwhelmingly positive feedback.
The Modulus ALIF is the latest addition to the NuVasive Advanced Materials Science (AMS) portfolio. It provides implants and instrumentation designed for both supine ALIF and XALIF procedures. The Modulus ALIF comes in a variety of sizes and lordotic options to accommodate differing patient anatomies.
The launch of Modulus ALIF is part of the company’s efforts to fuel continued growth and differentiation in the anterior spine segment. It is expected to leverage NuVasive’s position to become the market leader in ALIF. It is also intended to extend the Modulus portfolio across all procedural segments.
NuVasive will feature Modulus ALIF at the upcoming NuVasive Innovation Event and the 37th Annual Meeting of the Section on Disorders of the Spine and Peripheral Nerves.
Modulus ALIF in Detail
The Modulus ALIF technology features proprietary Modulus titanium design for enhanced osseointegration, biomechanical and imaging properties. The design also allows for enhanced visualization compared to solid titanium implants. Its porous surface structure permits participation in fusion and promotes new bone on-growth and in-growth. The technology features a zero-step locking mechanism to provide definitive tactile and visual confirmation, thereby instilling confidence in a surgeon's screw placement in the implant.
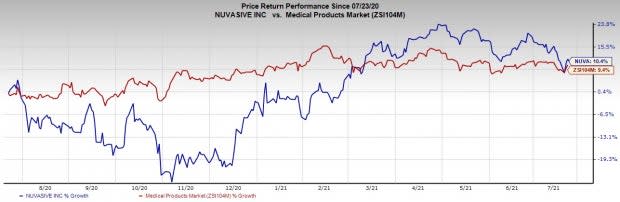
Image Source: Zacks Investment Research
The device's instrumentation is intended to support a surgeon's preferred surgical approach as it can be utilized in both supine ALIF and XALIF procedures. The instrumentation's low-profile and versatile features are designed to enhance operating room workflow efficiencies.
Industry Prospects
Per a report published in Allied Market Research, the global spinal implants and devices market is projected to witness a CAGR of 5.7% from 2020 to 2027. The factors contributing toward market growth during the forecast period may be attributed to a shift to minimally-invasive spine procedures, surge in elderly population, increasing prevalence of spinal disorders, and advancements in spine surgery. In addition, advancement in augmented and virtual reality, endoscopic surgery and 3D printed implants for minimally-invasive surgery is expected to fuel market growth further.
Given the market prospects, the recent launch of the Modulus ALIF seems well-timed.
Notable Developments
In June 2021, NuVasive announced that the Pulse platform received CE Mark authorization for its latest design update and clinical evaluations are underway in several countries throughout Europe. At present, Pulse is known to be the only enabling technology platform that has the ability to be utilized in 100% of spine procedures and throughout the entire operating room (OR) workflow. The latest CE Mark approval and clinical evaluations are stated to be key commercialization milestones for the Pulse platform.
In April 2021, the NuVasive Simplify Cervical Artificial Disc (Simplify Disc) received FDA approval for two-level cervical total disc replacement (cTDR). The Simplify Disc demonstrated clinical superiority to anterior cervical discectomy and fusion at 24 months in a two-level FDA Investigational Device Exemption study. The Simplify Disc is known to be one of the three devices approved for use in two-level cTDR procedures. The latest FDA go-ahead is further expected to broaden growth opportunities for the NuVasive C360 portfolio.
Share Price Performance
The stock has outperformed its industry over the past year. It has grown 10.4% compared to the industry’s 9.4% growth.
Zacks Rank and Key Picks
Currently, NuVasive carries a Zacks Rank #3 (Hold).
A few better-ranked stocks from the Medical-Products industry include Envista Holdings Corporation NVST, HillRom Holdings, Inc. HRC and BellRing Brands, Inc. BRBR, each sporting a Zacks Rank #2 (Buy). You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Envista Holdings has a long-term earnings growth rate of 26.4%.
HillRom Holdings has a long-term earnings growth rate of 7.9%.
BellRing Brands has a long-term earnings growth rate of 25.3%.
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
NuVasive, Inc. (NUVA) : Free Stock Analysis Report
HillRom Holdings, Inc. (HRC) : Free Stock Analysis Report
Envista Holdings Corporation (NVST) : Free Stock Analysis Report
BellRing Brands, Inc. (BRBR) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
