OPNT: Positive Results for OPNT003 PK Study; PD Results in 4Q21...
NASDAQ:OPNT
READ THE FULL OPNT RESEARCH REPORT
Business Update
Positive Results for OPNT003 PK Study
On July 6, 2021, Opiant Pharmaceuticals, Inc. (NASDAQ:OPNT) announced positive topline results from the confirmatory pharmacokinetic (PK) study of OPNT003, an intranasal (IN) formulation of nalmefene, which is being developed as a treatment for opioid overdose. This was an open label, randomized, crossover study in 68 healthy volunteers and compared 3mg IN nalmefene with 1mg intramuscular (IM) nalmefene.
The topline results showed that IN nalmefene achieved significantly higher plasma concentrations compared to the IM injection (P<0.0001). In addition, the time for IN nalmefene the achieve maximum plasma concentrations (Tmax) was consistent with what was seen in the previously completed pilot study, the maximum plasma concentration (Cmax) was higher than seen in the pilot study, and the plasma half-life of IN nalmefene was consistent with what was seen following other routes of administration (oral and parenteral).
Opiant had previously conducted an initial PK study of IN nalmefene that showed rapid increases in plasma levels with an onset faster than an IM injection along with a long half-life (6.7-7.8 hours). The following graph shows a rapid increase in nalmefene concentration following IN administration with and without INTRAVAIL®, which is a broad class of chemically synthesizable transmucosal absorption enhancement agents to allow the intranasal administration of therapeutics up to 30,000 Daltons molecular weight.

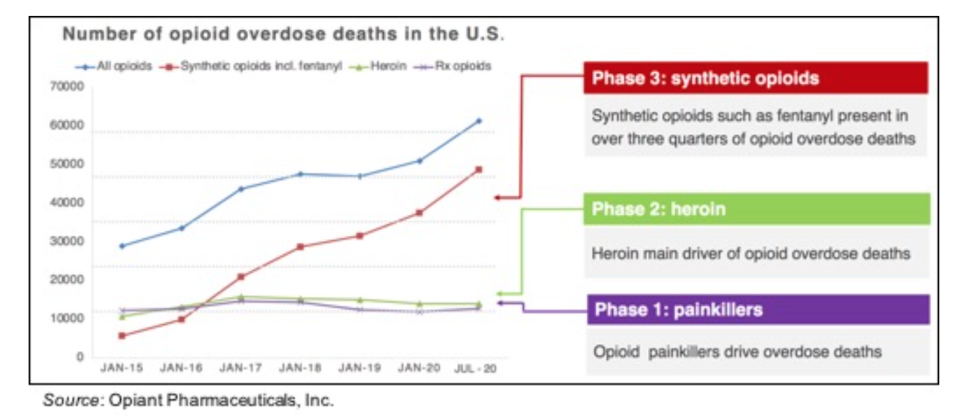
The following graph shows that the opioid epidemic currently ongoing in the U.S. is showing no signs of abating. The COVID-19 pandemic only exacerbated an already serious problem with opioid overdose deaths, which is now being fueled mostly by an increase in the use of synthetic opioids such as fentanyl, which has a half-life of more than seven hours (compared to 1-2 hours for heroin). For this reason, there is an urgent need for an opioid overdose therapy that is stronger and longer-acting than naloxone.
Compared to IN naloxone, IN nalmefene has a number of characteristics that make it superior as a treatment for fentanyl overdose, including increased affinity at the μ opioid receptors, a much greater half-life (which can help avoid re-narcotization), and a faster rate of absorption.

Opiant is currently conducting a pharmacodynamic (PD) trial of OPNT003 that will compare its effectiveness compared with IN naloxone. It is a single center, randomized, open label study in healthy volunteers that will test 3 mg nasal nalmefene to 4 mg nasal naloxone with the primary outcome examining the reversal of respiratory depression brought about by the synthetic opioid remifentanil (NCT04828005). We anticipate topline results from the trial in the fourth quarter of 2021.
The potential market opportunity for OPNT003 is substantial. Opiant is focused on four addressable markets: 1) the first responder market (EMTs, police force, fire dept.), which is key as they are typically the first person at the scene of an overdose; 2) both patients with opioid use disorder as well as their family members; 3) co-prescribing with opioid painkillers; and 4) civil defense, in which the government is concerned about the potential use of fentanyl as a chemical weapon and thus could stockpile nalmefene for use in the event of an attack.

New Chief Commercial Officer
On July 12, 2021, Opiant announced the appointment of Mr. Matthew Ruth as Chief Commercial Officer. Mr. Ruth has an extensive background in the opioid overdose space as he was responsible for building out the U.S. Operational, Commercial, Government Affairs, and Medical Affairs teams in preparation for the launch and commercialization of a branded nasal naloxone spray. His expertise will be a valuable addition for Opiant as the company moves toward the commercialization of OPNT003.
Conclusion
We’re glad to see that the results of the PK study matched or exceeded the results seen previously for IN nalmefene and we look forward to the results of the PD trial in the fourth quarter of 2021. The addition of Mr. Ruth is an important step for the company as the commercialization of OPNT003 gets closer and he seems particularly well suited to help the launch of OPNT003 be successful. With no changes to our model our valuation remains at $44.
SUBSCRIBE TO ZACKS SMALL CAP RESEARCH to receive our articles and reports emailed directly to you each morning. Please visit our website for additional information on Zacks SCR.
DISCLOSURE: Zacks SCR has received compensation from the issuer directly, from an investment manager, or from an investor relations consulting firm, engaged by the issuer, for providing research coverage for a period of no less than one year. Research articles, as seen here, are part of the service Zacks provides and Zacks receives quarterly payments totaling a maximum fee of $40,000 annually for these services. Full Disclaimer HERE.
