Deciphera (DCPH) Begins Dosing in Study on Cancer Candidate
Deciphera Pharmaceuticals, Inc. DCPH announced that it has dosed the first patient in a phase I study which is evaluating its investigational ULK kinase inhibitor, DCC-3116, for the treatment of patients with advanced/metastatic tumors driven by mutations in RAS/RAF genes.
Notably, the open-label, first-in-human study is investigating DCC-3116 as a single agent and also in combination with Mekinist (trametinib), an FDA-approved MEK inhibitor for treating advanced/metastatic tumors with a mutant RAS/RAF gene.
If data from this study is found to be positive, Deciphera plans to begin combination expansion cohorts which will evaluate DCC-3116 + Mekinist in patients with advanced/metastatic pancreatic ductal adenocarcinoma with KRAS or BRAF mutations; non-small cell lung cancer with KRAS, NRAS, or BRAF mutations; colorectal cancer with KRAS, NRAS, or BRAF mutations; and melanoma with NRAS or BRAF mutations.
Shares of Deciphera have plunged 35.8% so far this year against the industry’s increase of 1.2%.
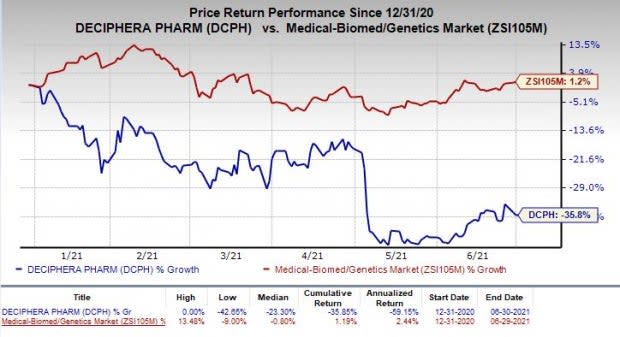
Image Source: Zacks Investment Research
Per the company, nearly one third of all cancers are driven by mutations in RAS/RAF genes. Hence, if successfully developed and upon potential approval, DCC-3116 can serve an area of high unmet medical need in the days ahead.
We remind investors that, Deciphera’s first commercial product, Qinlock (ripretinib), was approved by the FDA in May 2020, for the treatment of adult patients with advanced gastrointestinal stromal tumor (“GIST”) who have received prior treatment with three or more kinase inhibitors, including Novartis’ NVS Gleevec (imatinib).
The company is also working to expand the label of Qinlock for the larger commercial opportunity in second-line GIST.
The phase III INTRIGUE study is evaluating the efficacy and safety of Qinlock compared to Pfizer’s PFE Sutent (sunitinib) in patients with second-line GIST. Top-line data from the same is expected in the fourth quarter of 2021.
Zacks Rank& Stock to Consider
Deciphera currently carries a Zacks Rank #3 (Hold). A better-ranked stock in the healthcare sector is Repligen Corporation RGEN, with a Zacks Rank #2 (Buy) at present. You can see the complete list of today’s Zacks #1 Rank (Strong Buy) stocks here.
Repligen’s earnings estimates have been revised 18.3% and 14.7% upward for 2021 and 2022, respectively, over the past 60 days. The stock has rallied 4.2% year to date.
Zacks Names “Single Best Pick to Double”
From thousands of stocks, 5 Zacks experts each have chosen their favorite to skyrocket +100% or more in months to come. From those 5, Director of Research Sheraz Mian hand-picks one to have the most explosive upside of all.
You know this company from its past glory days, but few would expect that it’s poised for a monster turnaround. Fresh from a successful repositioning and flush with A-list celeb endorsements, it could rival or surpass other recent Zacks’ Stocks Set to Double like Boston Beer Company which shot up +143.0% in a little more than 9 months and Nvidia which boomed +175.9% in one year.
Free: See Our Top Stock and 4 Runners Up >>
Want the latest recommendations from Zacks Investment Research? Today, you can download 7 Best Stocks for the Next 30 Days. Click to get this free report
Novartis AG (NVS) : Free Stock Analysis Report
Pfizer Inc. (PFE) : Free Stock Analysis Report
Repligen Corporation (RGEN) : Free Stock Analysis Report
Deciphera Pharmaceuticals, Inc. (DCPH) : Free Stock Analysis Report
To read this article on Zacks.com click here.
Zacks Investment Research
